
Scientists find oceans of water on Mars. It's just too deep to tap. - Berkeley News
Seismic data from NASA's Insight lander indicate deep, porous rock filled with liquid water
Barsoom lives



i read that in a german mag, but i was too lazy to search for an english article. i also didn't know whwere to post it)
Scientists find oceans of water on Mars. It's just too deep to tap. - Berkeley News
Seismic data from NASA's Insight lander indicate deep, porous rock filled with liquid waternews.berkeley.edu
Barsoom lives



The Moon too it seems

Lunar mission data analysis finds widespread evidence of ice deposits
Deposits of ice in lunar dust and rock (regolith) are more extensive than previously thought, according to a new analysis of data from NASA's LRO (Lunar Reconnaissance Orbiter) mission. Ice would be a valuable resource for future lunar expeditions. Water could be used for radiation protection...phys.org
Asteroids may allow plants—so Mars’s moons could play a role early on.
In the 1950s, folks thought Martians would have green skin—laughable right?
Um…

Humans living on Mars could potentially turn green, biologist warns
Creating a colony on Mars would not only be technologically challenging, but also biologically taxing as well.www.unexplained-mysteries.com
that means we can get orion girls after some time
Humans living on Mars could potentially turn green, biologist warns
Creating a colony on Mars would not only be technologically challenging, but also biologically taxing as well.www.unexplained-mysteries.com

Indeed. Hydrogen is the most abundant element in the universe and oxygen is the third most abundant element, so the ubiquity of a simple compound of the two elements is unsurprising, especially in the outer solar system. Explaining how water got to Earth in the quantities we observe is more difficult. There's probably plenty on Mars, existing as permafrost and ice, although the low atmospheric pressure means it will sublime away over eons.Water is everywhere in the solar system. Actually being able to utilize it is the thing.
Water in the Universe (Astrophysics and Space Science Library Book 368) by Arnold HanslmeierDue to its specific chemical and physical properties, water is essential for life on Earth. And it is assumed that this would be the case for extra-terrestrial life as well. Therefore it is important to investigate where water can be found in the Universe. Although there are places that are completely dry, places where the last rainfall happened probably several 100 million years ago, surprisingly this substance is quite omnipresent. In the outer solar system the large satellites of Jupiter and Saturn are covered by a thick layer of ice that could be hiding a liquid ocean below.
This of course brings up the question of whether the recently detected extrasolar planets could have some water on their surfaces and how we can detect this. Water molecules are also found in interstellar gas and dust clouds.
This book begins with an introductory chapter reviewing the physical and chemical properties of water. Then it illuminates the apparent connection between water and life. This is followed by chapters dealing with our current knowledge of water in the solar system, followed by a discussion concerning the potential presence and possible detection of water on exoplanets. The signature of water in interstellar space and stars are reviewed before the origin of water in the Universe is finally discussed. The book ends with an appendix on detection methods, satellite missions and astrophysical concepts touched upon in the main parts of the book.
The search for water in the Universe is related to the search for extra-terrestrial life and is of fundamental importance for astrophysics, astrobiology and other related topics. This book therefore addresses students and researchers in these fields.

The average Martian surface temperature is 218K. From the diagram, you can see that at that temperature, there cannot be liquid water, however, you specified 277.15K. From the Clausius-Clapeyron relation, the required pressure for liquid water to form at 277.15K is 812.7 Pa. Given that the pressure on Mars ranges from 30 Pa to 1155 Pa (on Olympus Mons and in the Hellas Planitia respectively), and that the temperature on Mars can get as high as 300K in the summer, it is certainly possible to have liquid water on Mars temporarily at certain times of the Martian year.

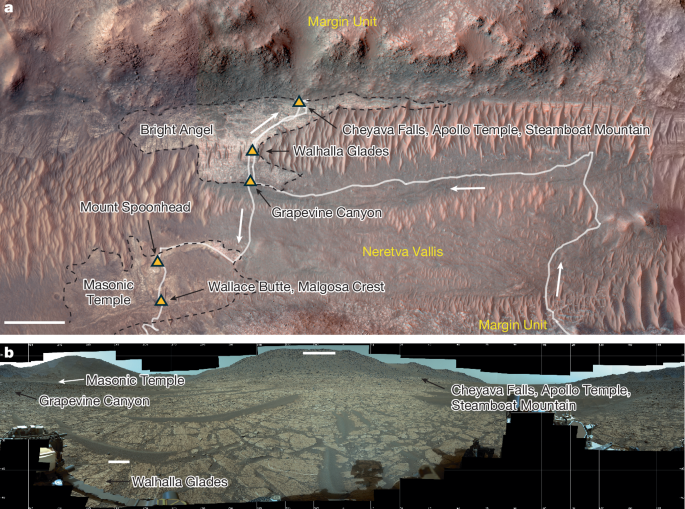
I didn't want to start a new topic when this was around. I hope this isn't too old to reply to.
With everything else going on, what would have been the biggest announcement of at least the year in some other alternate reality barely got notice this week:

Redox-driven mineral and organic associations in Jezero Crater, Mars - Nature
A geological, petrographic and geochemical survey of distinctive mudstone and conglomerate outcrops of the Bright Angel formation on Mars reveals textures, chemical and mineral characteristics, and organic signatures that warrant consideration as potential biosignatures.www.nature.com
NASA has more or less admitted what they found in Jezero Crater has no known origination except for bio processes. To be sure, after the Alan Hills asteroid debacle back in the late 90s, NASA has been terrified of claiming former Life on Mars, even as the evidence builds, and unless they get a disreputable fossil or bug-eyed monster, they're not going to. This is as close as NASA is going to admit.

This is a reasonable compromise:

Safeguarding the Earth from Martians: The Antaeus Report (1978-1981)
The story of spaceflight told through missions and programs that did not happen - that is, the great majority of them.spaceflighthistory.blogspot.com
More secure than the glove box in the movie LIFE.
We use essential cookies to make this site work, and optional cookies to enhance your experience.
