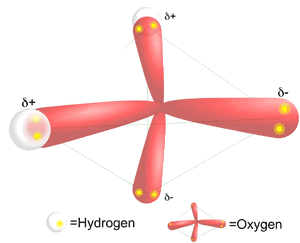
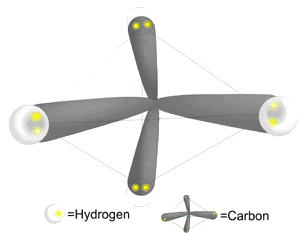
http://blogs.discovermagazine.com/badastronomy/2009/08/27/titanic-fog/
Well, at least now we know Earth isn't the only world in the universe with a climate cycle that affects some kind of liquid...
Well, at least now we know Earth isn't the only world in the universe with a climate cycle that affects some kind of liquid...